Apellai is raising an oversubscribed $8M Seed round at a $32M pre-money valuation, positioning the company for its next phase of regulatory and commercial growth. The round is led by Jameel Investment and Management Company (JIMCO), the global investment arm of the Jameel family, which established the Machine Learning in Health at MIT in 2018. JIMCO’s leadership role reinforces Apellai’s origins within the MIT research ecosystem and its credibility at the intersection of AI and healthcare. Additional investors include Village Global ($500M AUM early-stage fund backed by leading Silicon Valley founders), Grove Investment, and the Research Development & Innovation Authority (RDIA), which provides strategic regional support and access to non-dilutive funding. The company has also secured non-dilutive funding, strengthening both its research capabilities and capital efficiency.
Proceeds from the round will fund Apellai’s next major inflection point: transforming its two flagship AI platforms, Mirai for breast cancer and Sybil for lung cancer, from world-class academic models into fully regulated, reimbursable clinical products. Capital will be allocated toward Food and Drug Administration (FDA) compliance and regulatory filings, large-scale clinical validation, and software development for SaMD (Software as a Medical Device) certification. Funding will also support health-economic studies, deployment readiness, and sales enablement initiatives to establish commercial credibility with hospitals, payers, and enterprise imaging partners.
Apellai's upcoming joint venture (JV) with Paxera Health, a global imaging workflow company, will accelerate distribution and integration of its models into clinical environments worldwide. The partnership will provide immediate access to roughly 1,500 hospitals through Paxera’s existing network, enabling rapid deployment within established radiology and imaging workflows. This JV provides a scalable pathway to clinical adoption, positioning Apellai to transition from validation to widespread use across hospital systems.
The round provides approximately 24 months of operating runway under a capital-efficient plan that leverages partnerships and JVs for non-core infrastructure. This approach minimizes dilution while maximizing scalability, allowing Apellai to reach key regulatory, validation, and commercial milestones within the next two years and solidify its leadership in predictive oncology.
Apellai’s goal is clear: to establish its “AI to patient” pipeline as the gold standard for predictive oncology, creating a future where cancer can be identified and prevented years before it becomes visible.
More information on the terms of the round can be found in the ‘Capitalization & Current Raise’ Section below.
INVESTMENT TIMELINE
Virtual Company Presentation
Monday, October 20th, 2025
12:00 pm ET // 9:00 am PT
RSVP to Google Calendar invite
Call details available here
Final Investment Commitments Due
Friday, November 7th, 2025
We will take commitments on a rolling basis. To secure your allocation, please submit final commitments here.
Funding & Documents Due
Friday, November 14th, 2025
At the end of the commitment period, you will receive details regarding closing documentation and wiring instructions via Carta.
The Company's confidential financing documents and diligence materials are available for review in Carta. Please request access to data room materials at the top of the page. All documents are confidential and not for further distribution. Plum Alley Ventures reserves the right to not proceed with the investment opportunity if the $500,000 syndication minimum is not met. If you have specific questions or are interested in investing in Apellai, please contact us and submit your investment total here.
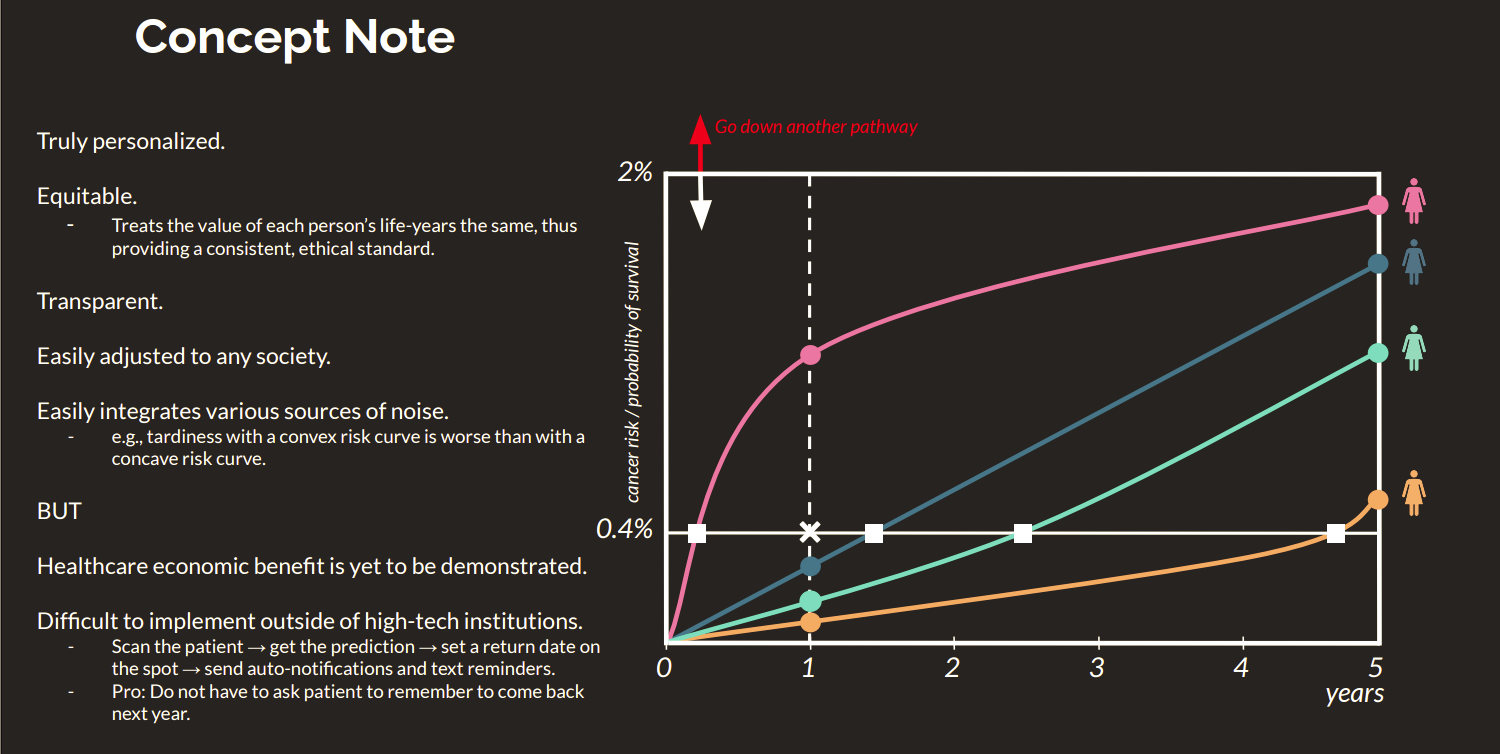
Cancer remains one of humanity’s greatest unsolved problems, and the gap between what is possible and what is practiced in early detection is enormous. Most cancers are only diagnosed once symptoms appear, when treatment is less effective and costs skyrocket. In breast and lung cancer, the two leading causes of cancer death, survival rates exceed 90% when detected early but drop drastically once the disease progresses. Yet current screening tools still fail to detect a large share of cases early enough. The consequence is measured not only in lives lost, but in tens of billions of dollars each year in avoidable treatment costs, hospitalizations, and productivity losses.
Existing solutions have not solved this problem because they were built for a different era of medicine. Traditional screening relies on static risk calculators and two-dimensional imaging interpreted by humans, both of which are limited by subjective judgment and coarse population averages. Tools like the Tyrer-Cuzick model were designed decades ago using narrow datasets and do not generalize well across diverse populations. Mammography, while widespread, can miss one in five cancers, especially in women with dense breast tissue. Low-dose CT scans for lung cancer have improved detection but often produce high false-positive rates, leading to unnecessary biopsies and follow-ups that burden both patients and health systems. These methods focus on identifying visible disease, not predicting who is most likely to develop it, which leaves an enormous blind spot in preventive care.
What sets Apellai apart is its ability to translate breakthrough research in the lab into real clinical impact. The company is building a full-stack “AI to patient” pipeline that unites model validation, regulatory compliance, and hospital deployment into one standardized process. This platform enables personalized screening intervals, earlier interventions, and better survival outcomes, making predictive oncology practical at scale. Its upcoming JV with Paxera Health provides direct access to roughly 1,500 hospitals, positioning Apellai for rapid adoption. With growing regulatory support, payer incentives for early detection, and cultural acceptance of AI in healthcare, Apellai is positioned to redefine cancer prevention. The company’s platform bridges the gap between cutting-edge research and real-world impact, delivering earlier detection, fewer false alarms, and a fundamental shift toward predictive, proactive care.
To learn more, watch FRONTLINE’s feature on the AI innovations developed by co-founders John Mikhael, MD, PhD, Diana Lu, MD, PhD, and Peter Mikhael, MSc below.
* Transformative Market Opportunity in Predictive Oncology: Cancer remains the leading cause of death worldwide, with breast and lung cancers representing two of the highest-mortality and highest-cost burdens in medicine. The US alone performs over 40M mammograms annually, yet current risk models and imaging tools routinely miss early-stage cancers or produce false positives that drive billions in unnecessary procedures. AI-enabled early detection can shift outcomes dramatically, as survival rates exceed 90% when breast cancer is detected early. Apellai directly targets this gap with predictive models designed to forecast individual risk up to six years in advance, reducing late diagnoses and healthcare inefficiency at population scale.
* Clinically Proven & Globally Validated AI Models: Developed and validated at MIT, Apellai’s flagship models, Mirai for breast cancer and Sybil for lung cancer, represent some of the most extensively validated predictive tools in oncology. Mirai has been tested on 2.5M patients across 70 hospitals in 21 countries, while Sybil has been validated on 85K CT scans across 20 hospitals in 11 countries. Across these diverse data sets, both models have consistently demonstrated exceptional accuracy and generalizability across age, ethnicity, and imaging modalities. This global validation has established Apellai’s platform as one of the most rigorously tested AI screening technologies in medicine, providing a strong foundation for prospective clinical trials, regulatory submission, and eventual integration into standard clinical practice.
* Full-Stack “AI to Patient” Infrastructure for Clinical Deployment: Apellai is building the first vertically integrated platform that takes AI models from research to patient use in hospitals. The platform standardizes data ingestion, model validation, software lifecycle management, and regulatory documentation under Good Machine Learning Practices (GMLP). It integrates seamlessly with existing Picture Archiving and Communication System (PACS) and EHR systems, allowing for rapid hospital onboarding without workflow disruption. This end-to-end infrastructure is designed to scale globally while maintaining compliance with FDA, Conformité Européenne (CE), and future Medical Device Reporting (MDR) requirements, positioning Apellai as both a technology and regulatory enabler for clinical AI adoption.
* Robust Commercial Model with Proven Reimbursement Pathways: Revenue will initially come from hospital subscriptions and per-scan fees during the out-of-pocket phase, followed by payer reimbursement once Current Procedural Terminology (CPT) code coverage is secured. Similar AI imaging tools, such as Optellum’s lung nodule analysis platform, have already achieved Centers for Medicare & Medicaid Services (CMS) reimbursement of approximately $700 per scan, validating the economic model. Apellai’s health-economic simulations project strong Quality-Adjusted Life Year (QALY)-based returns, given its ability to reduce false positives, lower follow-up costs, and prevent late-stage treatments. The company’s long-term vision includes a SaaS platform for third-party AI model integration, leveraging its validated “AI to patient” infrastructure to create a multi-application ecosystem.
* Accelerating Regulatory & Commercial Momentum: Apellai is advancing regulatory submissions for FDA and CE approval with early pilot programs scheduled over the next 12 to 18 months. Partnerships with national health systems in the United Kingdom (UK), the United Arab Emirates (UAE), the Kingdom of Saudi Arabia (KSA), and Taiwan are supporting real-world validation and early adoption. These pilots are designed around FDA-aligned endpoints and reimbursement-oriented data collection. As the platform demonstrates measurable reductions in missed cancers and unnecessary biopsies, Apellai will have the data and infrastructure needed to drive large-scale payer integration and population-level screening adoption.
* Strategic Advantage: Apellai’s partnership with JIMCO creates a powerful bridge between MIT’s leadership in medical AI and a global investment platform with deep healthcare reach. JIMCO’s backing provides Apellai with direct access to world-class research, top technical talent, and deployment pathways spanning the Middle East, Europe, and Asia. At the same time, AI in healthcare is transitioning from experimental to indispensable, with regulators fast-tracking safe, explainable models and payers incentivizing preventive tools that reduce system-wide costs. Apellai’s technology, evidence base, and go-to-market infrastructure position it to capture this momentum at precisely the right inflection point. The company’s predictive models address two of the largest oncology markets globally while laying the foundation for expansion into pancreatic and ovarian cancers. With a capital-efficient structure and strong strategic partnerships, Apellai is poised to set the global standard in predictive oncology and reshape how the world approaches cancer prevention.

Cancer is the leading cause of death worldwide, and breast and lung cancers remain among the most lethal due to late detection and inconsistent screening, representing an estimated $40B serviceable addressable market (SAM) in the United States (US) alone. Each year, more than 40M mammograms are performed across the country, yet traditional tools still miss a significant number of early-stage cancers. Nearly half of women diagnosed with breast cancer have no known risk factors, while outdated screening methods produce false negatives or trigger costly and unnecessary follow-ups. The result is not only lives lost but also billions in preventable healthcare costs and a growing burden on already strained systems.
Early detection dramatically improves survival rates by over 90% and reduces the financial and emotional toll of treatment, yet innovation in cancer screening has lagged behind advances in other areas of medicine. The tools most commonly used today, such as risk calculators, mammograms, and low-dose Computed Tomography (CT) scans, remain limited in both precision and reach. Risk models like Tyrer-Cuzick often underperform across diverse populations, while mammography can miss up to one in five cancers, particularly in women with dense breast tissue. Low-dose CT scans for lung cancer, though effective in some cases, frequently generate false positives that lead to costly and invasive follow-ups. These inefficiencies drive billions in avoidable healthcare spending every year and perpetuate disparities in early detection outcomes across racial and socioeconomic groups.
However, AI is reaching a critical point of clinical maturity. Deep learning models can now extract subtle biomarkers from existing imaging data that are invisible to the human eye, allowing for risk prediction years before symptoms arise. Regulators such as the FDA are increasingly approving AI-based diagnostic tools, and payers are beginning to reimburse their use, recognizing the potential for earlier intervention and cost savings. The convergence of urgent medical need, technological capability, and supportive policy creates a once-in-a-generation opportunity to transform how cancer is detected, predicted, and ultimately prevented.
Apellai’s approach marks a fundamental shift in how cancer is understood and managed. Rather than relying on human interpretation, its AI models learn from millions of imaging and clinical data points to uncover risk patterns that even the most experienced radiologists cannot detect. These are not incremental improvements but step-change leaps forward in predictive medicine. Mirai and Sybil analyze routine mammograms and CT scans to forecast a patient’s cancer risk five to six years before tumors become visible, giving clinicians the ability to personalize screening schedules, intervene earlier, and prevent unnecessary procedures.
Validated across millions of patients and over 70 hospitals in 21 countries, including leading institutions such as Massachusetts General Hospital (US), Karolinska University Hospital (Sweden), Chang Gung Memorial Hospital (Taiwan), and Asan Medical Center (South Korea), both models have demonstrated exceptional accuracy and consistency across age groups, ethnicities, and imaging modalities. This proven performance makes Apellai’s technology one of the most rigorously tested and broadly generalizable in oncology AI. Its predictive capability is more than a technical success. It is a breakthrough in equitable healthcare, ensuring that earlier detection and better outcomes are possible for patients everywhere, regardless of geography or background.
Beyond the technology itself, Apellai is solving the hardest operational problem in AI healthcare: translating cutting-edge AI research into regulated, clinically adopted products that physicians can trust. The company’s “AI to patient” platform unites model validation, regulatory compliance, and hospital deployment within a single standardized pipeline. This end-to-end approach ensures that AI tools meet the stringent quality, security, and workflow requirements needed for real-world use. Combined with extensive global validation, payer-aligned economic modeling, and a leadership team experienced in both science and commercialization, Apellai’s strategy positions it to succeed where others have stalled, making early cancer prediction practical, scalable, and transformative.
The market conditions have never been more favorable. Regulatory agencies such as the FDA are fast-tracking AI-enabled medical tools, while CMS has already established reimbursement frameworks for AI-driven lung risk assessments, reaching up to $700 per scan. Payers are actively searching for innovations that lower costs without compromising outcomes, and health systems are under pressure to improve efficiency as cancer incidence continues to rise. With validated models, scalable infrastructure, and growing institutional acceptance of AI in medicine, Apellai stands ready to define a new era of predictive oncology, bringing the right technology to market at precisely the right time to set new standards of care.

Apellai’s Predictive Oncology Platform
Apellai is pioneering a new paradigm in cancer care: AI-driven prediction rather than detection. The company’s platform uses deep learning to forecast a patient’s risk of developing breast or lung cancer years before the disease becomes visible, enabling earlier intervention, personalized screening, and a major reduction in unnecessary procedures and costs.
At the heart of Apellai’s platform are two flagship models, each representing a breakthrough in how medical imaging can be used for preventive medicine rather than diagnostic triage.

How the Platform Works
Mirai: AI-Powered Breast Cancer Risk Prediction
Mirai is a deep learning model that analyzes routine mammograms to predict a woman’s one- to five-year risk of developing breast cancer. Instead of mimicking a radiologist’s detection process, Mirai identifies subtle, distributed imaging signals across both breasts that are invisible to the human eye. This predictive capability allows clinicians to stratify patients by risk and personalize screening intervals, supplemental imaging, and preventive interventions.
Peer-reviewed studies have demonstrated Mirai’s performance advantage over legacy risk models such as Tyrer-Cuzick and prior deep learning baselines, with reported five-year Area Under the Curve (AUCs) ranging from 0.66-0.71 and one-year AUCs in the 0.79-0.84 range. These results show stronger discrimination, better calibration across subgroups, and sustained robustness across age, race, and imaging systems.

Mirai’s Generalizability & Scale
Mirai has been validated on over 2.5M mammograms from more than 70 hospitals across 21 countries. This global validation demonstrates consistency across demographic, geographic, and technical boundaries, addressing the calibration bias that limits many existing risk models. Such scale establishes Mirai as one of the most rigorously tested AI systems in breast imaging and provides a foundation for future regulatory filings and clinical adoption.
Sybil: AI Model for Early Lung Cancer Prediction
Sybil is a low-dose CT (LDCT)-based deep learning model that forecasts a patient’s six-year risk of developing lung cancer from a single baseline scan, even when no nodules are visible. The model identifies complex risk signatures that go beyond what radiologists can detect, addressing a key limitation of current LDCT protocols that depend on visible nodule size or morphology.
In validation studies, Sybil achieved near-term discrimination of approximately 0.90+ AUC at one to two years, tapering to 0.75-0.79 by year six, which compares favorably to traditional clinical predictors. Validation across more than 85K CT scans, 20 hospitals, and 11 countries demonstrates Sybil’s cross-site generalization and consistent performance in both academic and community health systems.

Why It Matters
Both Mirai and Sybil address the same core problem: current cancer screening methods detect disease too late and rely on limited visual cues rather than predictive risk modeling. By forecasting cancer years before it becomes radiologically visible, Apellai enables risk-adjusted screening that is earlier, more precise, and more equitable. These tools have the potential to save lives, reduce false positives and unnecessary follow-ups, and lower the long-term financial burden on health systems. Their proven generalizability makes them equally valuable in high-resource and underserved settings.
Platform Architecture & Roadmap
Apellai’s “AI to Patient” platform provides an end-to-end system for deploying predictive AI safely and at scale. The stack combines GMLP standards, bias and explainability controls, controlled retraining, International Organization for Standardization (ISO)-aligned software lifecycle management, and post-market surveillance. The platform is built for Health Insurance Portability and Accountability Act (HIPAA) and General Data Protection Regulation (GDPR) compliance and integrates seamlessly with PACS and EHR systems.
Near-term priorities include advancing Mirai and Sybil through FDA and CE approvals, hardening clinical QA (Quality Assurance) / QC (Quality Control) processes, and supporting early pilots in partnership with leading health systems. Subsequent versions, Mirai 2.0 and Sybil 2.0, will incorporate longitudinal imaging data and expanded modalities, with future products targeting pancreatic and ovarian cancer as well as cross-cutting applications such as incidental detection, trial enrichment, and companion diagnostics.
Commercial Model & Access Strategy
Apellai’s business model is structured for efficient scaling across both enterprise and consumer healthcare channels. The company’s primary revenue will come from a combination of annual hospital subscriptions and per-scan fees during the initial, out-of-pocket phase. As regulatory approvals are secured, Apellai will transition toward reimbursement-based adoption through CMS and private payer frameworks already in place for comparable AI imaging tools.
Short-term monetization will focus on:
Long-term growth will come from payer and CMS reimbursement for risk-stratification and early detection. The pathway is already validated through Optellum’s AI-assisted lung nodule management tool, which received CMS reimbursement at ~$600-700 dollars per scan. Once reimbursement is established, Apellai expects strong margins and recurring revenue, supported by a capital-light, cloud-native infrastructure that scales like enterprise software.
Economic Rationale
Apellai’s health-economic modeling shows that earlier detection and fewer false positives can substantially reduce treatment costs while improving patient outcomes. The company’s models deliver clear QALY gains and positive return on investment even at modest adoption levels. Lower downstream treatment costs and more precise screening translate into savings for payers and hospitals while improving survival rates.
This alignment of economic efficiency and clinical value positions Apellai to benefit from the global transition toward value-based care and risk-adjusted reimbursement. By reducing both diagnostic waste and late-stage treatment costs, Apellai creates measurable value for every stakeholder in the cancer care continuum.
Clinical Validation & Real-World Readiness
Apellai’s traction is grounded in extensive clinical validation and real-world performance data.
Both models have shown strong performance across age, ethnicity, and imaging modalities, ensuring equitable outcomes across diverse populations. Apellai is extending this validation through pilot collaborations with national health systems in the UK, Taiwan, and the UAE or KSA region. These studies will generate prospective clinical evidence and support payer engagement and reimbursement readiness.
Go-To-Market Strategy
Apellai’s commercialization strategy follows a two-phase approach that balances early traction with long-term scalability.
Phase 1: Regulatory and Pilot Execution (0-18 months)
Phase 2: Market Expansion (18-36 months)
This phased approach allows Apellai to generate early commercial traction, validate performance in real-world environments, and expand globally with minimal infrastructure costs.
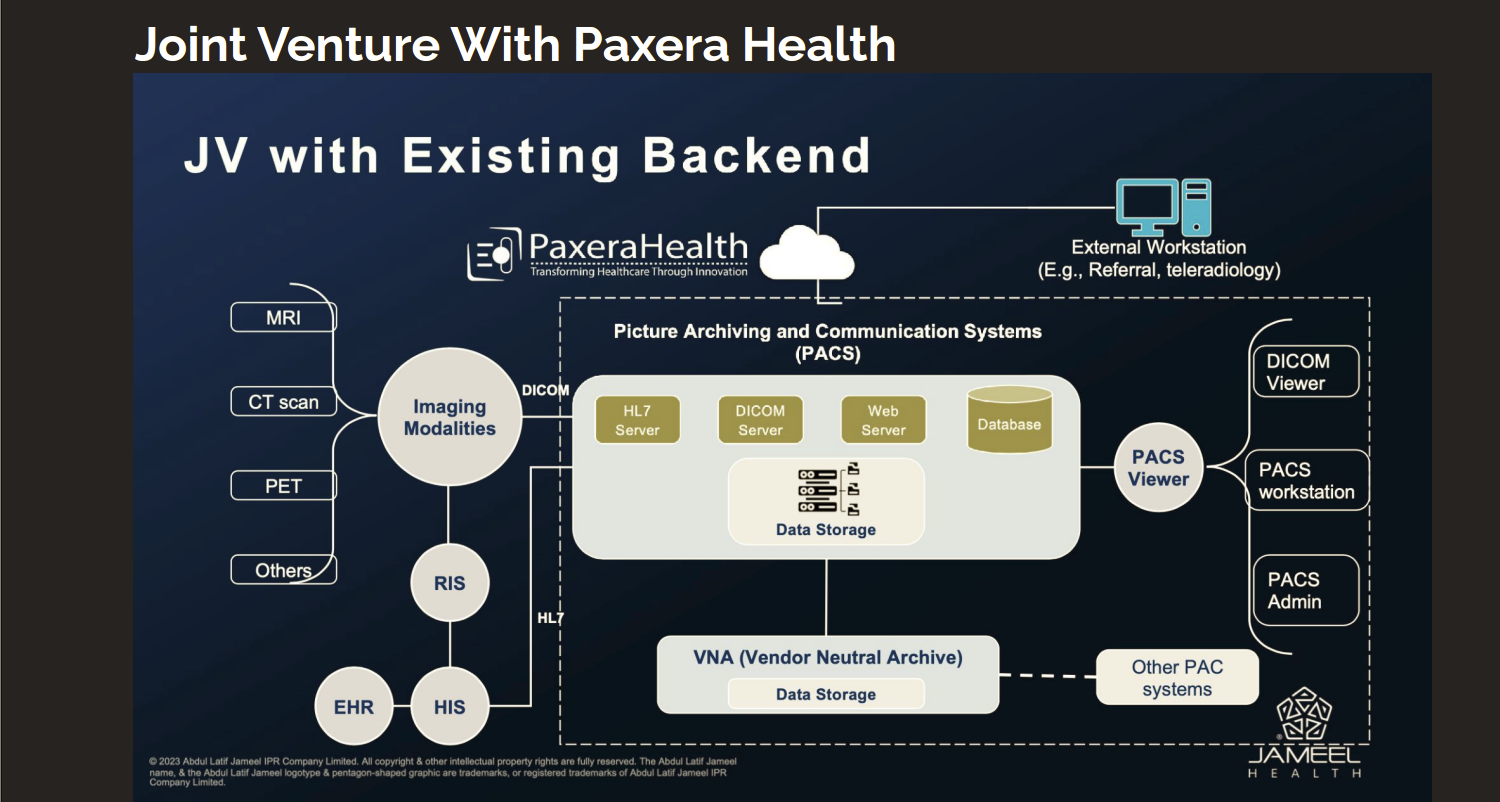
Strategic JV with Paxera Health
Apellai has formed a JV with Paxera Health, a global leader in enterprise imaging and workflow software used by more than 1,500 hospitals worldwide. The partnership will accelerate the commercial rollout of Apellai’s predictive oncology platform by embedding its AI models, Mirai and Sybil, directly into Paxera’s imaging ecosystem, allowing clinicians to access cancer risk predictions seamlessly within existing radiology workflows.
The JV gives Apellai immediate global distribution and regulatory leverage through Paxera’s FDA-cleared software infrastructure and established data compliance network across the Middle East, Europe, and North America. This integration enables rapid clinical deployment, collection of real-world validation data, and streamlined engagement with payers and regulators.
By leveraging Paxera’s infrastructure, sales channels, and support teams, Apellai can scale globally without building costly distribution systems, maintaining a capital-efficient growth model. The partnership creates a fast, compliant path to market and positions Apellai to make predictive cancer screening accessible at scale, transforming hospital imaging networks into proactive, AI-enabled centers for early detection.
Traction & Strategic Momentum
Apellai’s progress demonstrates both technical credibility and commercial readiness.
Apellai’s capital-efficient model targets breakeven once reimbursement adoption begins. Its software-based architecture and scalable cloud delivery create a high-margin foundation for long-term growth. The company’s combination of global validation, regulatory readiness, and operational discipline positions it as the most advanced contender to commercialize predictive oncology at scale.
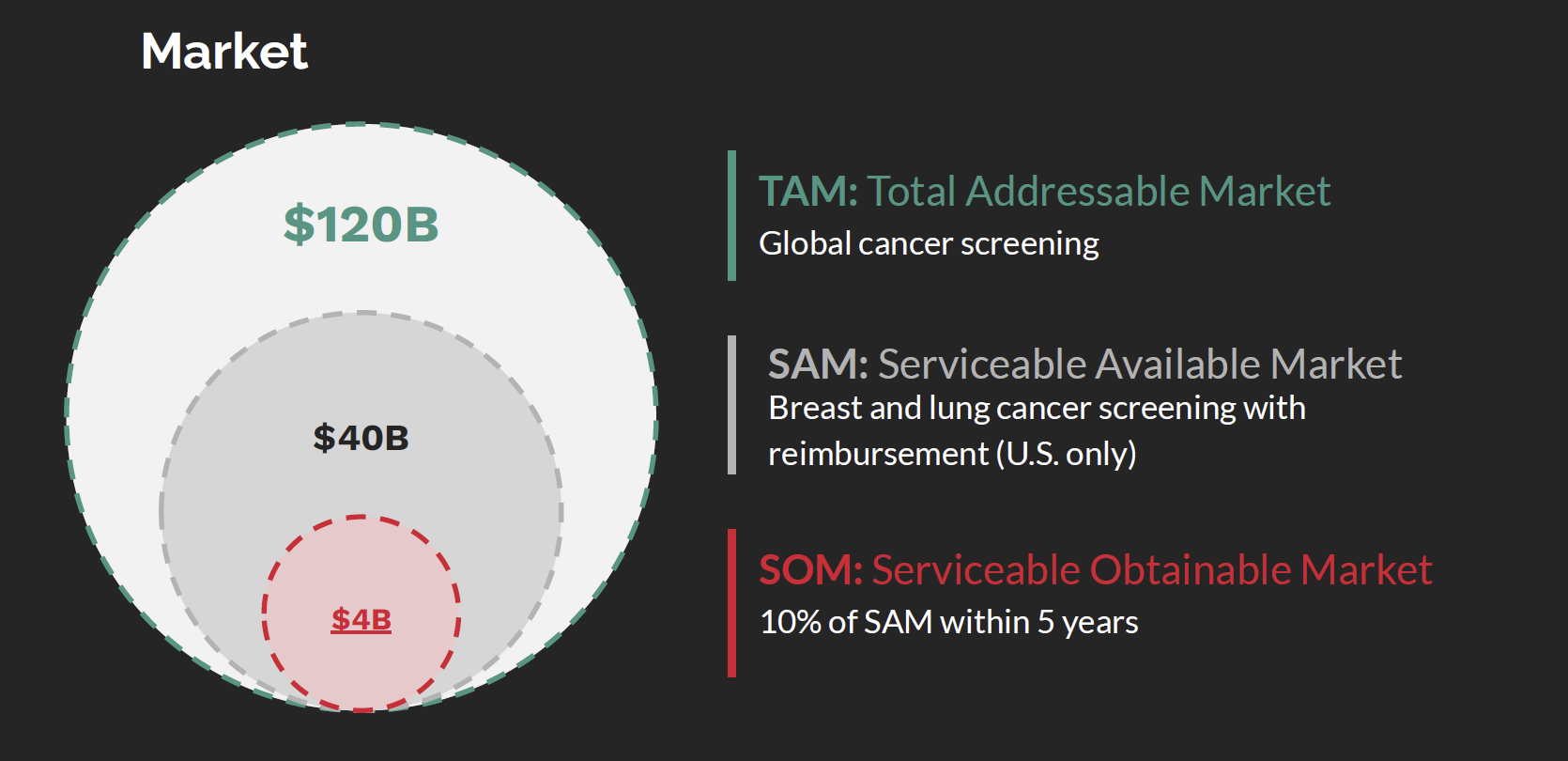
The global cancer screening market represents a $120B TAM, with a $40B SAM in breast and lung cancer screening reimbursable in the US Apellai is targeting 10% SOM of ~$4B within 5 years. Importantly, Mirai and Sybil can be applied retroactively to existing scans (up to 5-6 years old), dramatically expanding addressable volume.
Market Dynamics
Cancer screening is one of the largest and most rapidly expanding areas in global healthcare. Growth is driven by aging populations, increasing cancer incidence, and the urgent need for earlier and more accurate detection. Worldwide, cancer care spending surpassed $250B in 2025, with early detection technologies representing a growing portion of that expenditure.
The financial and clinical rationale for predictive screening is compelling. Early-stage treatment can cost up to five times less than late-stage care, and survival rates improve dramatically when disease is caught earlier. Despite this, most screening approaches remain rooted in visual detection and static risk models that fail to identify disease early enough. The adoption of AI-based risk prediction is accelerating because it sits at the convergence of three powerful forces:
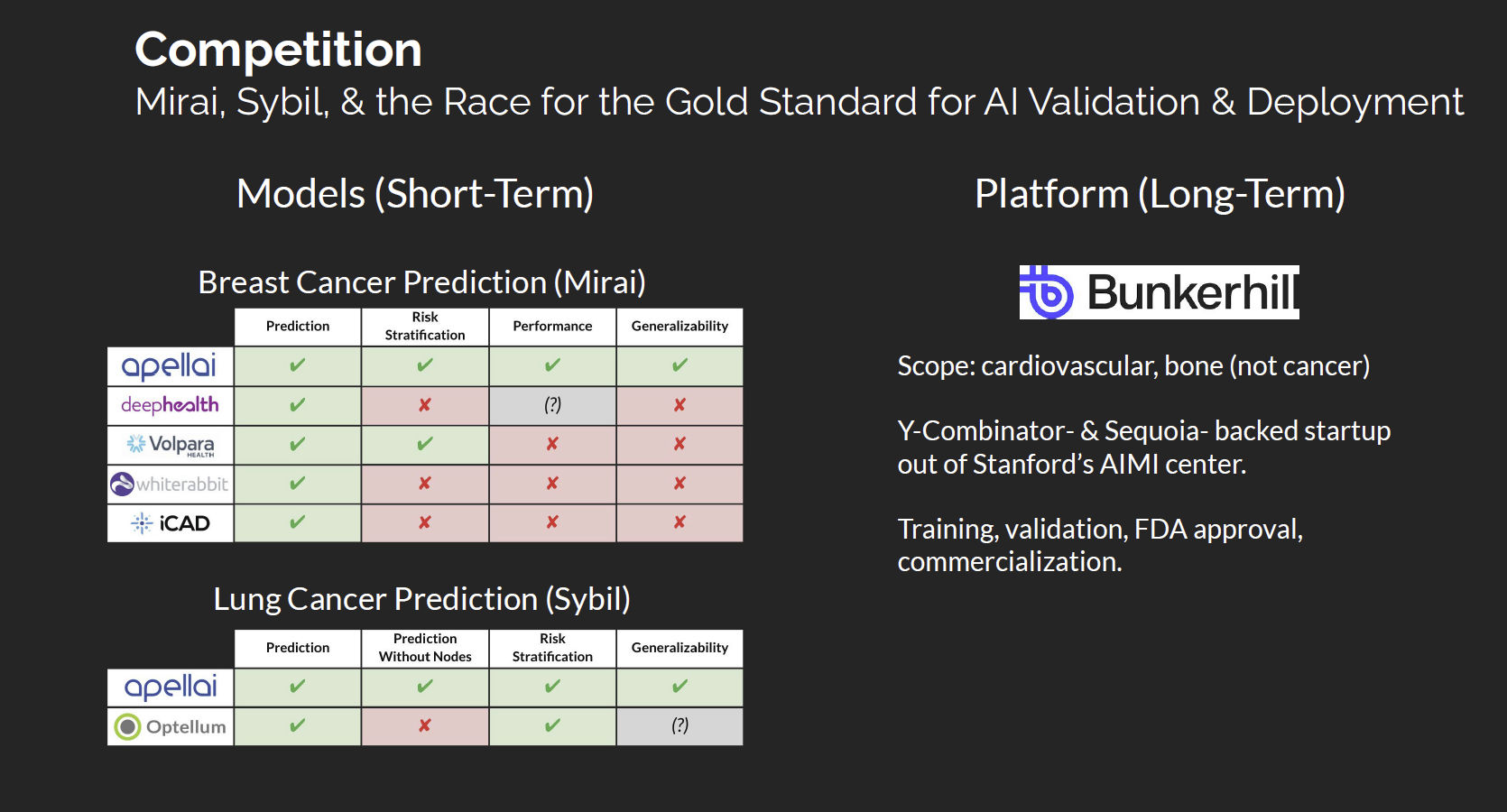
Competitive Landscape
The current AI imaging landscape remains fragmented, with most players focused on real-time detection rather than long-term risk prediction. Companies such as HeartFlow, Viz.ai, Aidoc, and iCAD have built strong clinical distribution networks but primarily assist radiologists in interpreting existing disease, not forecasting its onset. Academic tools like Google’s LYNA and Lunit INSIGHT MMG also remain detection-focused and often lack large-scale validation or regulatory infrastructure for clinical deployment.
Apellai stands apart by focusing on prediction and risk stratification rather than detection. Its models, Mirai and Sybil, have been validated across millions of patients in multiple countries, demonstrating robust generalizability and consistent performance across diverse populations. The company is developing a complete predictive oncology platform that integrates validated AI models, regulatory compliance, and deployment infrastructure capable of supporting reimbursement and real-world use.
By addressing both breast and lung cancer (the two largest oncology screening markets), Apellai has established a first-mover advantage that is reinforced by scale, regulatory readiness, and demonstrated accuracy across global populations. In parallel, Apellai will expand research and development (R&D) into additional high-impact cancer indications, such as pancreatic and ovarian, broadening its predictive oncology platform beyond breast and lung cancer.
Strategic Positioning
Apellai operates at the intersection of clinical need, regulatory readiness, and economic efficiency. Its platform fills a critical gap between imaging-based detection tools and traditional risk calculators, offering a solution that is both data-driven and clinically actionable.
From a regulatory perspective, the company’s adherence to SaMD and GMLP standards positions it ahead of peers in readiness for FDA and CE clearance. Commercially, Apellai’s software integrates seamlessly with existing hospital systems through PACS and EHR interoperability, minimizing workflow disruption and enabling rapid adoption.
Economically, Mirai and Sybil align with value-based care initiatives by allowing payers and health systems to target high-risk populations, improve outcomes, and reduce the burden of unnecessary procedures. Through this combination of clinical value, regulatory credibility, and economic logic, Apellai is emerging as a leader in predictive oncology and a key player in the evolution of precision screening.

Major Inflection Points
Apellai is currently completing an oversubscribed $8M Seed round at a $32M pre-money valuation, which positions the company for a 24-month runway and significant regulatory and commercial progress. The round is led by JIMCO , with participation from Village Global, Grove Investment, and the RDIA. JIMCO brings deep ties to healthcare innovation and the MIT Jameel Clinic, which originally incubated the Mirai model, while Village Global provides strategic access to a global technology and AI network. Grove Investment adds experience in early-stage life sciences, and RDIA’s involvement brings strong regional support and non-dilutive capital linked to strategic data partnerships.
The oversubscribed round reflects strong investor confidence in Apellai’s early traction, global partnerships, and rapid technical progress. Alongside its equity investors, the company has secured non-dilutive funding tied to data access and clinical collaborations. This capital structure supports the company’s goal of converting Mirai and Sybil from validated academic models into regulated, reimbursable products, while minimizing dilution and maintaining flexibility for future rounds.
A large portion of the raise will fund regulatory preparation, product development, and clinical validation as Apellai advances Mirai and Sybil toward FDA and CE clearance. The company is implementing ISO 13485 quality systems and post-market surveillance while accelerating technical development to potentially skip version 1.5 and move directly to version 2.0, which will integrate longitudinal imaging, multimodal data, and advanced risk calibration. Capital will also support prospective studies and pilot programs with health systems in the United Kingdom, Taiwan, and the Middle East, alongside health-economic research to strengthen reimbursement dossiers and payer engagement.
The company is also advancing a series of strategic partnerships that significantly expand its footprint and data access. Apellai is in active collaboration with Lean Business Services, a Public Investment Fund-owned entity that oversees roughly 70% of Saudi Arabia’s healthcare data infrastructure. This partnership will enable deployment of Apellai’s platform within the Saudi Ministry of Health and Ministry of Interior systems. The company is also working with Mayo Clinic’s AI research team to extend Sybil’s predictive modeling beyond oncology into non-oncologic disease prediction. Additional deployment partnerships are under discussion with California imaging centers, M42 in the UAE, and health organizations in Turkey and Japan, along with exploratory conversations with pharmaceutical companies for clinical trial applications.
As part of its regional expansion strategy, Apellai plans to establish Apellai KSA, a Saudi-based entity headquartered in Riyadh. This move addresses data sovereignty requirements and demonstrates the company’s commitment to building regional capacity and technical infrastructure. Saudi Arabia will serve as the core hub for development and model curation, while the UAE will act as the initial commercialization hub due to its more advanced regulatory and digital health environment. The strategic plan is to anchor development in Saudi Arabia, build local capability with Saudi institutions, and deploy through the UAE to reach Europe and adjacent markets.

Dr. John Mikhael, Co-Founder and CEO:, John is a physician-scientist with deep expertise at the intersection of neuroscience, oncology, and AI. He earned his MD and PhD in Computational Neuroscience from the Harvard-MIT Program in Health Sciences and Technology, after completing an MSc in Neuroscience at Oxford University as a Rhodes Scholar and a BSc in Mathematics at MIT. His research has been published in leading journals including Cell, Current Biology, and Neuropsychopharmacology, focusing on computational modeling of biological learning and uncertainty. Before founding Apellai, John served as a teaching fellow in computational cognitive neuroscience at Harvard and remains an advisor on several distinguished fellowship committees at MIT. At Apellai, he leads the company’s scientific and strategic direction, translating advanced AI research into regulated, clinically deployable oncology products.
Dr. Diana Lu, Co-Founder and COO: Diana is a physician-scientist and experienced operator with a background in cancer biology and global innovation management. She received her MD and PhD from the Harvard-MIT Program in Health Sciences and Technology, where her doctoral research focused on transcriptional regulation in pediatric sarcomas, with first-author publications in Nature Cell Biology and Cancer Cell. She previously worked as a research associate at the Broad Institute and directed the MIT Global Startup Workshop, where she built partnerships to advance science-based ventures across emerging markets. Diana oversees Apellai’s operations, partnerships, and global commercialization strategy, combining scientific rigor with executional discipline to scale the company’s predictive oncology platform.
Peter Mikhael, Co-Founder and Head of Research: Peter is a computer scientist and engineer specializing in the application of deep learning to medical imaging and biological systems. He is completing his PhD in Electrical Engineering and Computer Science at MIT, where he also earned his MSc under the supervision of faculty at the Jameel Clinic for Machine Learning in Health. Peter is the co-inventor of both Mirai and Sybil, Apellai’s flagship cancer risk-prediction models, and has co-authored multiple peer-reviewed publications in Nature Chemical Biology, Science Translational Medicine, Scientific Reports, and Journal of Clinical Oncology. His research on mammography and CT-based cancer prediction has been validated across multi-institutional datasets, forming the technical foundation of Apellai’s platform. At the company, Peter leads product development, research, and engineering, guiding the transition from academic prototypes to secure, scalable, regulator-grade AI systems.
Apellai is guided by a world-class advisory board spanning AI, clinical oncology, health policy, and commercialization, including leaders from MIT, Berkeley, Harvard, and CVS Health. With expertise across AI, radiology, and healthcare operations, this group brings the scientific, clinical, and industry insight needed to translate Apellai’s technology from the lab to global clinical adoption.

Financing & Runway Sensitivity: The current Seed round provides approximately two years of runway based on planned regulatory filings and pilot deployments. Delays in regulatory approval, slower-than-expected reimbursement progress, or expansion into additional cancer indications could accelerate capital needs. While investor interest has been strong, sustained execution will require disciplined spending and timely follow-on financing. Market conditions or extended timelines could necessitate bridge capital, adjustments to hiring plans, or partnership-driven funding to maintain momentum.