This opportunity is reserved for our existing member investors that have invested in EpiBone’s previous 2017 and 2021 rounds. For clarification, this round is not available to all Plum Alley members.
EpiBone is raising an insider-led $4M Series A-1 financing at a $105M pre-money valuation, co-led by Kendall Capital Partners and Tech Council Ventures. This round introduces pay-to-play mechanics and a progressive Bonus Preferred share structure, supporting its new strategic pivot into medical tourism to further the bone stem cell technology we previously invested in.
Key terms for the pay-to-play round with a minimum Plum Alley Ventures Company SPV investment of $250,000 include:
Please note that for our member SPV to participate, we will need a minimum of $250,000 in member investment commitments. If this threshold is not reached, Plum Alley Ventures Company reserves the right not to participate in the current Series A-1 pay-to-play round, resulting in our current Preferred shares converting to Common on a 10:1 basis.
More information on the terms of the round can be found in the ‘Capitalization & Current Raise’ Section below.
INVESTMENT TIMELINE
Virtual Company Presentation
Monday, September 8th, 2025
12:00 pm ET // 9:00 am PT
RSVP to Google Calendar invite
Call details available here
Final Investment Commitments Due
Thursday, October 2nd, 2025
We will take commitments on a rolling basis. To secure your allocation, please submit final commitments here.
Funding & Documents Due
Thursday, October 9th, 2025
At the end of the commitment period, you will receive details regarding closing documentation and wiring instructions via Carta.
The Company's confidential financing documents and diligence materials are available for review in Carta. Please request access to data room materials at the top of the page. All documents are confidential and not for further distribution. If you have specific questions or are interested in investing in EpiBone, please contact us and submit your investment total here. Given the Syndication timeline to meet EpiBone's Series A-1 closing date, if the Plum Alley Ventures Company Syndicate does not meet certain milestones it may not move forward.
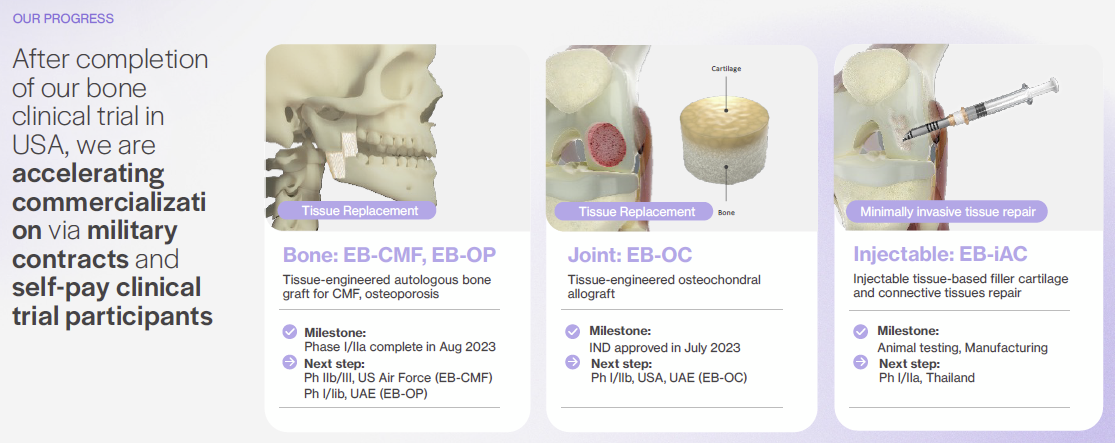
EpiBone has advanced its platform across bone, joint, and cartilage with key milestones in three strategic markets:

The company is now focused on development and growth of medical tourism in these markets, with the UAE and Thailand as strategic anchors.
Proceeds will fund expansion in the UAE, including a GMP facility in Abu Dhabi, a Phase III program, and the Living Joint Replacement Program. Capital will also advance three EB-CMF implantations with the USAF, complete injectable cartilage EB-iAC human implantations in Thailand, and secure osteoporotic bone (EB-OP) IND approval in the UAE. The UAE and Thailand are top medical tourism destinations, which supports cost-recovery sales during Phase II and accelerates time to revenue ahead of FDA approval.
Co-founded by Chief Executive Officer (CEO) Dr. Nina Tandon (PhD, Columbia) and Chief Science Officer (CSO) Dr. Sarindk “Ik” Bhumiratana (PhD, Columbia; Postdoc, Columbia), EpiBone pairs deep tissue-engineering expertise with foundational intellectual property (IP) for its living bone and cartilage platform. Their track records, supported by a board and advisors that include Moderna co-founder Robert Langer, PhD and KKR’s co-founder Henry Kravis, underpin strong scientific and execution credibility.
In our lifetime, three out of four people will live with parts of their bodies they were not born with. Nowhere is this more evident than in orthopedics, where more than 10M bone and cartilage reconstruction procedures are performed annually in the United States (US) alone. Current approaches, such as metal hardware, synthetic substitutes, and scarce cadaver grafts, offer structure but fall short on true biological integration. At the same time, advances in regenerative medicine and tissue engineering over the past decade have opened the door to solutions that heal, remodel, and endure.
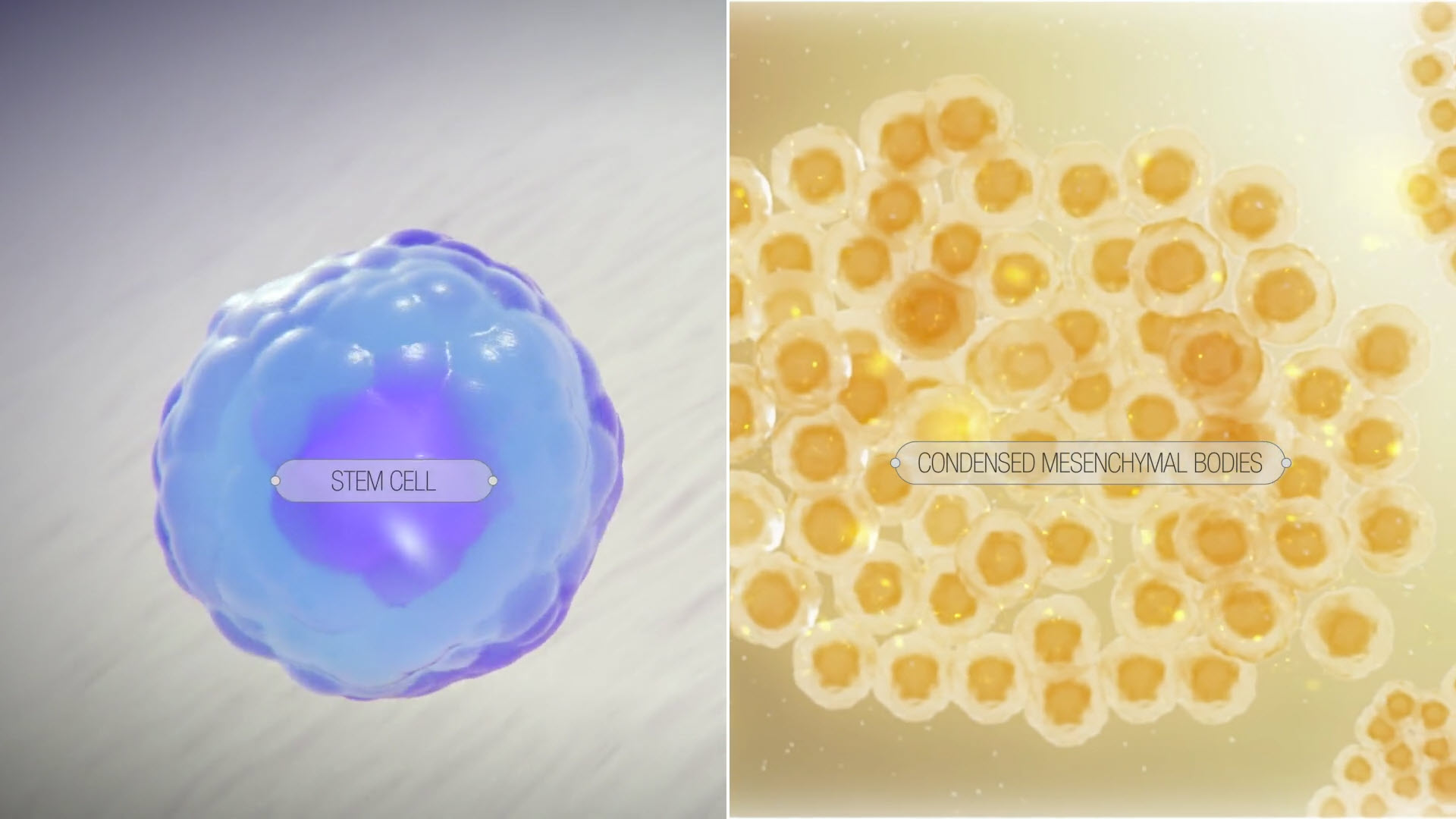
EpiBone is pioneering that future. As a clinical-stage regenerative medicine company, EpiBone engineers living bone and cartilage customized to each patient using their own stem cells. By delivering patient-specific biologic implants that integrate and remodel over time, the company aims to reduce the complications, limitations, and failure rates of today’s standard interventions. EpiBone brings together 3D imaging, computational design, advanced biomaterials, and perfusion bioreactors to create functional tissue that is ready at implantation. Its platform spans multiple indications, positioning the company to address high-burden conditions in the jaw, knee, hip, and other joints.
EpiBone’s closed-loop tissue engineering platform mimics natural skeletal development. Computed tomography (CT) imaging defines an exact anatomical scaffold, autologous adipose-derived stem cells are expanded and seeded, and perfusion bioreactors deliver oxygen, nutrients, and mechanical cues to mature living grafts. This technology underpins a pipeline that covers bone, joint, and cartilage products:
The company has already completed early clinical work for EB-CMF in the US, holds an Investigational New Drug (IND) clearance to begin EB-OC Phase I/IIb trials in the US, and is preparing to initiate EB-iAC human studies in Thailand.
To accelerate adoption and revenue, EpiBone is executing internationally through hubs in the UAE and Thailand. The UAE provides access to destination orthopedic care, strong intellectual property protection, and modern logistics, and EpiBone is pursuing conditional approval, building GMP capacity in Abu Dhabi, and launching the Living Joint Replacement Program. Relationships include Hub71, Cleveland Clinic Abu Dhabi, Burjeel Holdings (which has committed $2.5M in in-kind support for an osteoporosis trial), and the UAE Department of Health. Thailand provides the second anchor for EB-iAC, with leading hospitals, experienced contract research organizations, and a thriving medical tourism sector. Together, these hubs allow earlier patient access, cost-recovery revenue, and strategic partnerships, including planned EB-CMF implantations with USAF.
EpiBone’s regulatory and financing roadmap is built to convert international traction into lasting US leadership. The company’s global trials are being designed with FDA-aligned endpoints, and EpiBone plans to present this data in a Type C meeting in late 2026 or early 2027 to accelerate the US approval pathway. Its differentiation lies in full anatomical matching, the use of autologous stem cells, and a platform adaptable across multiple skeletal sites - advantages that clearly distinguish EpiBone from drug therapies, non-personalized synthetics, and single-indication cartilage products. With multiple Investigational New Drugs (INDs) already cleared, human data advancing, and insider participation in the current Series A-1 financing ahead of a targeted Series B following the Type C meeting, EpiBone is well positioned to lead the next era of regenerative skeletal medicine.

To learn more, watch the recent update presented to Plum Alley Ventures Company by co-founders Nina Tendon, PhD and Ik Bhumiratana, PhD below.
* Urgent Unmet Need and Market Pull: Orthopedic regeneration faces clear supply and outcomes gaps. ~2.2M procedures use bone grafting each year with roughly 500K in the US while only ~3K fresh osteochondral allografts are available annually. In the US, more than 30M adults live with osteoarthritis. EpiBone targets this demand with patient-matched, living grafts that aim to integrate and remodel.
* Ex-US Launch Engine: EpiBone is building a clinical-commercial beachhead in Abu Dhabi to accelerate time to first revenue. Relationships include Hub71, Cleveland Clinic Abu Dhabi (exploratory), Burjeel Holdings (letter of support for $2.5M in-kind for the osteoporosis trial), and the UAE Department of Health for conditional approval and grants. Thailand anchors EB-iAC first-in-human work with established CRO infrastructure and medical-tourism logistics.
* Clinical and Regulatory Momentum Across Three Programs: EB-CMF (autologous bone) has completed a US Phase I/IIa; EB-OC (osteochondral autograft) holds US IND clearance to begin a Phase I/IIb; EB-iAC (injectable cartilage) is advancing to human implantation in Thailand. The mix of autologous and allogeneic products expands addressable use cases from cranio-maxillofacial reconstruction to focal knee lesions and low-grade cartilage defects.
* Differentiated Tissue-Engineering Platform: The platform emulates developmental biology: CT-guided design, a custom scaffold, and adipose-derived stem cells matured in perfusion bioreactors that deliver oxygen, nutrients, and controlled mechanical cues. The result is living, patient-specific bone, cartilage, or osteochondral grafts built to fit precisely and remodel in vivo, addressing the fit, durability, and supply limitations of synthetics and cadaver allografts.
* Defense and Regulatory Catalysts: The USAF is sponsoring EB-CMF implantations for craniofacial repair, creating high-need validation and an early customer channel. International trials in the UAE and Thailand are being designed with FDA-aligned endpoints, with plans to present foreign data at a future Type C meeting. The UAE Department of Health process and Burjeel’s in-kind support further de-risk execution and early adoption.
EpiBone’s platform recreates key steps of skeletal development to grow living grafts that match each patient’s anatomy. For personalized bone and osteochondral constructs, the workflow begins with a CT scan of the defect and a small adipose biopsy to isolate mesenchymal stem cells. Engineers design a patient-specific scaffold and matching perfusion bioreactor, then culture the cells under controlled oxygen, nutrients, and mechanical loading to drive maturation. Typical culture time is about 3 weeks for bone and about 4 weeks for cartilage, at which point the graft is ready for implantation.
The platform supports three modalities. EB-CMF is a patient-specific bone graft for cranio-maxillofacial defects that has shown maintenance of structure, integration, and vascular infiltration in large-animal studies prior to human trials. EB-OC is a bone-cartilage composite grown as cartilage on a bone scaffold for joint repair. EB-iAC is an injectable, off-the-shelf cartilage product with scalable manufacturing and multi-month shelf life for on-demand use and easy shipping.
What makes it different
EpiBone’s grafts are living and designed to integrate with surrounding tissues, unlike metal or ceramic hardware that provides structure but not regeneration. The company’s IP stack covers bioreactors, osteogenic and chondrogenic media, MSC processing, next-generation scaffolds, automation, injectable cartilage compositions, and delivery tools, creating a defensible process and manufacturing moat.
Two FDA-cleared INDs enable ongoing progression of EB-CMF and EB-OC, while EB-iAC advances internationally with FDA-aligned endpoints. This staged path generates early clinical experience and revenue outside the US while building the dataset for the US approval path.
EpiBone’s platform emulates developmental biology: imaging defines the exact anatomy, a custom scaffold is fabricated, and the patient’s adipose-derived stem cells are seeded and matured in a perfusion bioreactor that delivers oxygen, nutrients, and controlled mechanical cues. This produces living, patient-specific grafts for bone, cartilage, or composite osteochondral repair.
How The Platform Works
Why It Matters
The approach targets three long-standing pain points in surgery: precision fit, biologic integration, and supply. Patient-matched geometry reduces intra-op carving, living tissue supports remodeling and long-term durability, and engineered constructs avoid the severe scarcity of cadaver osteochondral allografts.
Product Portfolio
Takeaway
EpiBone runs a digital, traceable workflow from imaging through scaffold fabrication and perfusion bioreactor culture that enables scale-out manufacturing of personalized or batch allogeneic products. By uniting CT-guided design with autologous or banked cells in perfusion bioreactors, the platform aims to deliver living skeletal grafts that fit precisely, integrate biologically, and expand access where donated tissue is scarce. The EB-CMF, EB-OC, and EB-iAC programs translate this into focused indications where each element of the technology provides a practical advantage in the operating room and beyond.
EpiBone operates a staged commercialization plan that starts ex-US and scales into the US after approval. Near term, the company plans conditional clinical use in the UAE and Thailand so it can treat patients during Phase 2 and later, generate early revenue on a cost-recovery basis, and collect FDA-aligned outcomes while it builds a GMP site in Abu Dhabi. Post-approval in the US, EpiBone intends to price against complex reconstructive procedures and pursue coverage with evidence packages built from its international and US data. The platform supports both autologous, patient-specific grafts for bone and osteochondral repair and an allogeneic injectable cartilage product that can be stocked and scheduled flexibly.

The UAE is the near-term anchor given its position as a global health-care hub. Dubai alone welcomed more than 691K international health tourists in 2023, generating over AED 1B (~$280M) in spending. The country offers strong intellectual property protection, modern logistics that simplify cold-chain distribution, and progressive regulators open to conditional approval. EpiBone expects the UAE to enable revenue three to five years faster than a US-only path, while expanding the effective total addressable market (TAM), treating more patients sooner, and reducing equity capital requirements.

Thailand provides the second anchor for EB-iAC clinical translation. Ranked consistently among the world’s top medical tourism destinations, Thailand attracted 3.5M foreign patients annually pre-COVID and has rebounded strongly with government-backed initiatives. The country combines leading university hospitals, established contract research organizations (CROs), and a reputation for affordable, high-quality care. For EpiBone, this ecosystem offers efficient patient recruitment, cost-effective trial execution, and a natural launchpad for its injectable cartilage product.
Together, the UAE and Thailand create an international engine that enables earlier patient access, cost-recovery revenues, and FDA-relevant data collection. These efforts are designed to generate pre-Biologics License Application (BLA) revenue abroad, then scale domestically once FDA approval is secured. A BLA is the formal submission to the FDA requesting authorization to market a biologic in the United States, supported by clinical, manufacturing, and quality data.
Key achievements include:
Near-term milestones (18-24 months):
Market Dynamics
Disease Burden Across Pipeline
Strategic Positioning
EpiBone’s platform grows living, patient-specific bone and cartilage from a person’s own adipose-derived stem cells, matured in custom perfusion bioreactors to match the exact anatomy captured by CT imaging. This approach aims to combine full anatomical fit with biologic integration and remodeling, a differentiated position relative to synthetics and cadaveric grafts.
In markets like knee cartilage repair, where procedure demand vastly exceeds allograft supply, and in CMF reconstruction, where precise form and function matter, EpiBone’s autologous, patient-matched grafts target clear pain points in access, fit, and long-term durability.
Three Major Inflection Points
EpiBone raised an $8M Seed round in December 2019 at a $27M pre-money valuation, led by Tech Council Ventures with participation from Amgen Ventures, Hackensack Meridian Health, and Henry Kravis. Capital supported IND clearance and first-in-human work for EB-CMF while scaling the perfusion bioreactor platform. In March 2024, the company introduced a $5M Pre-Series B bridge with a 30% discount to the Series B to begin ex-US expansion and advance EB-CMF, EB-OC, and EB-iAC toward next milestones. In November 2024, EpiBone closed a convertible note round led by Kendall Capital Partners with Lifespan Vision Ventures and EMV Capital to accelerate global clinical operations from Abu Dhabi.
In July 2025, EpiBone launched an insider-led Series A-1 Preferred of up to $4M equal to the prior Series A price, co-led by Kendall Capital Partners and Tech Council Ventures. The structure is pay-to-play with a 10 to 1 reverse split of existing preferred into common prior to the round and “pull-through” restoration for investors taking pro rata. A progressive Bonus Preferred schedule incentivizes investments above a certain level.
With $43M in equity financing and $3.9M in non-dilutive grants reported through early 2024, the company now adds additional non-dilutive support from a 2025 Air Force Research Laboratory award of about $1.25M to validate EB-CMF for maxillary use, strengthening the US defense revenue path.
Proceeds will:
The 2024 Kendall Capital-led note and the 2025 AFRL contract provide strategic, non-dilutive fuel that de-risks execution and creates a clear runway to a targeted Series B. The plan is milestone-driven: generate initial revenue in the UAE and the US defense channel, complete Thailand EB-iAC first-in-human, and advance through UAE and US regulatory gates. International studies are being built with FDA-aligned endpoints, with the intent to present the data in a late-2026 or early-2027 Type C meeting to shape and accelerate the US approval path.

Pictured from left: Co-Founders Nina Tandon, PhD and Ik Bhumiratana, PhD
Dr. Nina Tandon, Co-Founder and CEO: Nina holds a PhD in biomedical engineering from Columbia, completed a postdoctoral fellowship in stem cells and tissue engineering at Columbia, and earned an Executive MBA in healthcare entrepreneurship from Columbia Business School. She is a TED Senior Fellow, a former McKinsey consultant, and an MIT alum in electrical engineering. Fast Company named her one of the 100 most creative people in business, and her TED talk on personalized medicine has surpassed 1M views.
Dr. Sarindr “Ik” Bhumiratana, Co-Founder and CSO: Trained at Columbia University for both his PhD and postdoctoral fellowship, Ik pioneered methods to engineer bone, cartilage, and osteochondral tissues from stem cells, work that underpins EpiBone’s platform. He is listed on two patents, has authored more than 15 peer-reviewed papers and four book chapters, and now leads translation of the science into clinical products and trials. Industry coverage credits his leadership in optimizing perfusion bioreactors to mimic in-vivo growth conditions for living grafts.
Upon discussion with the founders, EpiBone put together their view of how and where the company could exit. The following information is presented for illustrative purposes only and contains numerous assumptions made by EpiBone which may or may not occur. Investors should perform their own assessment, and are cautioned that milestones mentioned below may or may not be achieved.
