As a clinical-stage biotechnology company, Juvena Therapeutics is pioneering a new class of protein-based biologics derived from stem cell secretomes. Juvena’s mission is to unlock the therapeutic potential of secreted proteins to treat a wide range of chronic and rare degenerative diseases. By combining advancements in human stem cell biology, quantitative proteomics, and AI-powered drug discovery, Juvena has created a proprietary discovery engine (JuvNET) that rapidly identifies and develops disease-modifying biologics in a fraction of the time and cost of traditional approaches.
At the core of Juvena’s approach is the creation of a functional map of regenerative biology, linking specific secreted proteins to disease-modifying phenotypes across organ systems. This platform has already produced a rich and growing pipeline, including lead programs for neuromuscular disease, obesity, osteoarthritis, fibrosis, and metabolic liver disease. Juvena has also built one of the world’s most comprehensive libraries of stem-cell-derived secreted proteins, many of which are novel and previously uncharacterized.
Juvena’s platform has already achieved significant industry validation. The company recently entered a strategic partnership of $650M+ with Eli Lilly focused on muscle and metabolic indications. Lilly also became a strategic investor in Juvena’s current oversubscribed $40M Series B raise, co-leading the round alongside new institutional investor Jefferson Life Sciences. With clinical programs advancing, a validated AI discovery engine, and robust investor interest, Juvena is positioned to lead a new era in protein-based precision therapeutics.
To learn more, watch the recent update presented to Plum Alley Ventures Company by co-founders Hanadie Yousef, PhD and Jeremy O’Connell, PhD below.
* Large Market Potential with De-Risked Regulatory Pathways: Juvena is targeting a large and underserved market of muscular dystrophy associated with neuro degeneration and obesity-oriented diseases. The company’s platform has broad applications across the $161B rare disease market, which is projected to grow at a 13.1% CAGR through 2028. By harnessing secreted proteins - naturally occurring, tissue-specific signaling molecules - Juvena’s biologics offer a superior safety and efficacy profile compared to small molecules, gene therapies, or antibodies.
* Validated AI-Driven Drug Discovery Engine (JuvNET) with Robust IP: At the heart of Juvena’s platform is JuvNET, a proprietary AI-powered discovery engine built to unlock the therapeutic potential of secreted proteins. By integrating high-throughput phenotypic screening, quantitative proteomics, and advanced ML, JuvNET systematically identifies and prioritizes proteins with disease-modifying potential. This end-to-end platform rapidly ranks candidates by predicted efficacy, enabling high-confidence selection and significantly compressed development timelines. Since inception, JuvNET has analyzed over 2,000 secreted proteins, validated 55 across six therapeutic areas, and progressed eight into in vivo models - a testament to the platform’s scalability and precision. It also recently announced its $650M multi-target discovery partnership with Eli Lilly, focused on muscle and metabolic diseases. This partnership both commercially validates Juvena’s discovery engine and sets the stage for broader application across pharma pipelines.
* First-Mover in Secreted Protein Therapeutics: Juvena is the first clinical-stage biotech systematically developing secreted protein-based biologics, representing a breakthrough in regenerative medicine. Unlike small molecules, antibodies, or gene therapies, secreted proteins, especially those derived from human stem cells, are naturally potent, tissue-specific, and designed by evolution to communicate between cells and orchestrate repair. These properties translate into better safety profiles, improved tissue targeting, and more profound therapeutic potential.
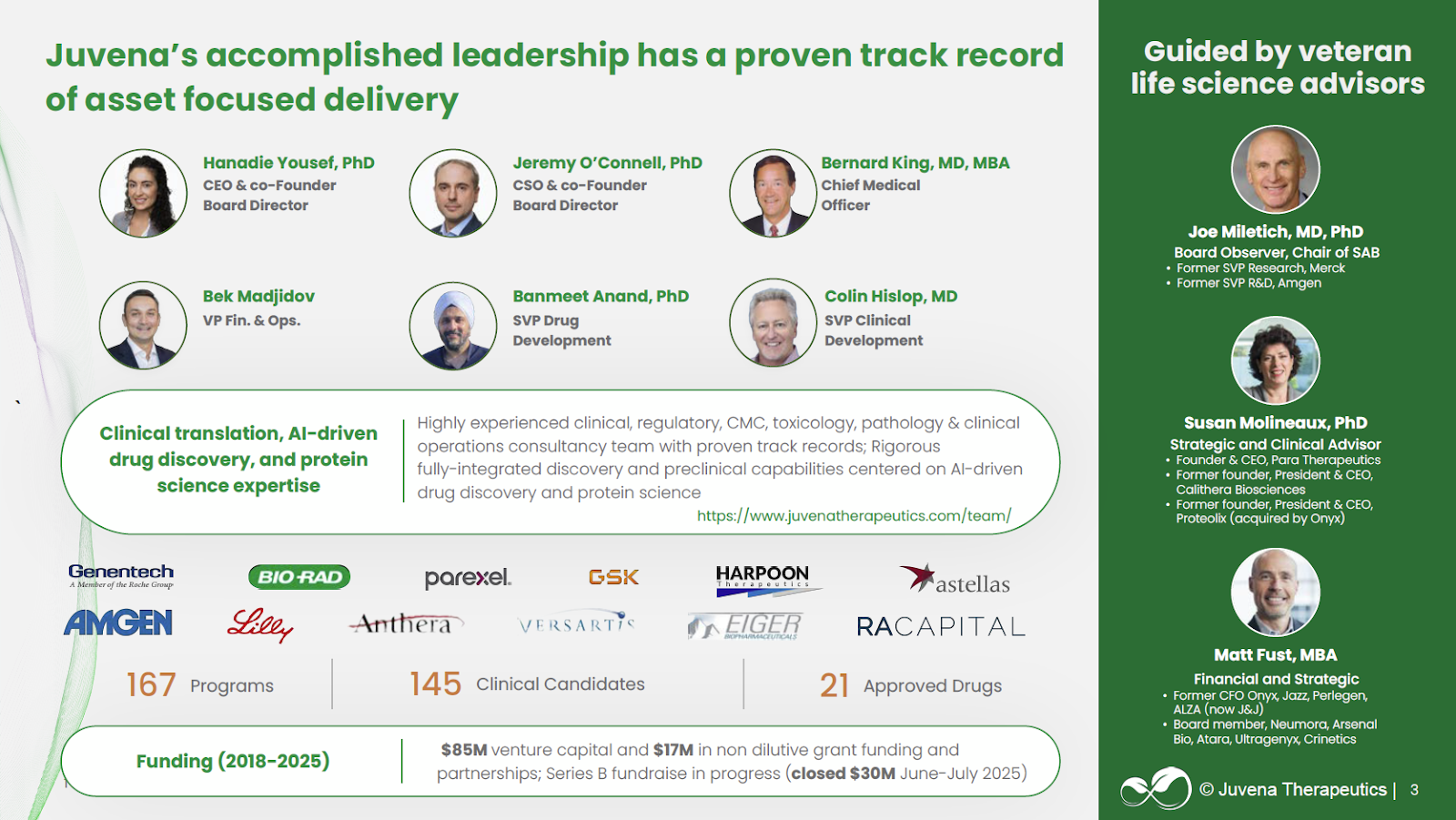
* Exceptional Scientific and Operational Leadership: Juvena is led by a seasoned, cross-functional team with deep experience across drug development, protein engineering, and AI/ML. CEO & Co-Founder Hanadie Yousef, PhD (UC Berkeley, Stanford) is a pioneer in aging and regenerative biology. CSO & Co-Founder Jeremy O’Connell, PhD (Harvard) brings deep proteomics and computational biology expertise. Additionally, the scientific advisory board and team include senior leaders from Merck, Amgen, Jazz, and Calithera.
* Deep-Pocketed and Prestigious Investors: Juvena has attracted a world-class syndicate spanning venture capital, strategic pharmaceuticals, and institutional science-backed funds. Key investors include prior lead investors Mubadala Capital (sovereign wealth fund of Abu Dhabi) and Horizons Ventures alongside new investor Jefferson Life Sciences (part of $2.5B AUM Jefferson River Capital, the family office of former President & COO of Blackstone). Eli Lilly recently entered as a strategic investor, co-leading Juvena’s Series B with a $6M equity investment alongside a 3-year, $650M multi-target R&D partnership. Other notable investors include Jeff Dean, Head of Google AI, furthering confidence in Juvena’s ML-powered discovery engine. To date, Juvena has raised $85M in total funding, including the current oversubscribed $40M Series B raise.
Juvena is raising an oversubscribed $40M Series B financing at a $71M pre-money valuation to advance its mission of transforming the treatment of degenerative diseases through secreted protein biologics. The company has already closed its initial target of $30M, which was increased to accommodate the expanded investor interest. As existing investors into the prior Series A round, we were able to secure additional allocation into the current Series B raise. With the Company’s advancements in clinical outcome, strategic pharma partnership with Eli Lilly and capitalization by key investors, Plum Alley Ventures Company is excited to present this as a new investment opportunity to all Plum Alley Members.
The company was co-founded by CEO Dr. Hanadie Yousef (PhD, UC Berkeley; Postdoc, Stanford) and Chief Science Officer (CSO) Dr. Jeremy O’Connell (PhD, UT-Austin; Postdoc, Harvard), whose complementary expertise in aging biology, regenerative signaling, proteomics, and machine learning (ML) laid the foundation for the company’s platform. Together, they envisioned a new therapeutic paradigm: one that harnesses the regenerative power of secreted proteins to treat complex, chronic, and rare diseases.
The proceeds of this round will fund Phase 1/2 proof-of-concept studies for Juvena’s lead program, a first-in-class biologic enhancing muscle regeneration and metabolism in myotonic dystrophy type 1 (DM1) and other myopathies. Funding will also advance an obesity and metabolic disease candidate with a novel, energy-expenditure-driven mechanism and expand Juvena’s AI-enabled JuvNET discovery platform through its strategic partnership with Eli Lilly. Valued at over $650M in potential milestones over the next three years, this collaboration validates the platform’s capabilities and will identify new muscle-metabolic targets for obesity, further broadening Juvena’s pipeline and commercial reach.
More information on the terms of the round can be found in the ‘Capitalization & Current Raise’ Section below.
INVESTMENT TIMELINE
Virtual Company Presentation
Monday, August 18th, 2025
12:00 pm ET // 9:00 am PT
RSVP to Google Calendar invite
Call details available here
Final Investment Commitments Due
Friday, August 29th, 2025
We will take commitments on a rolling basis. To secure your allocation, please submit final commitments here.
Funding & Documents Due
Friday, September 5th, 2025
At the end of the commitment period, you will receive details regarding closing documentation and wiring instructions via Carta.
The Company's confidential financing documents and diligence materials are available for review in Carta. Please request access to data room materials at the top of the page. All documents are confidential and not for further distribution. If you have specific questions or are interested in investing in Juvena Therapeutics, please contact us and submit your investment total here. Given the expedited Syndication timeline to meet Juvena's Series B closing date, if the Plum Alley Ventures Company Syndicate does not meet certain milestones it may not move forward.
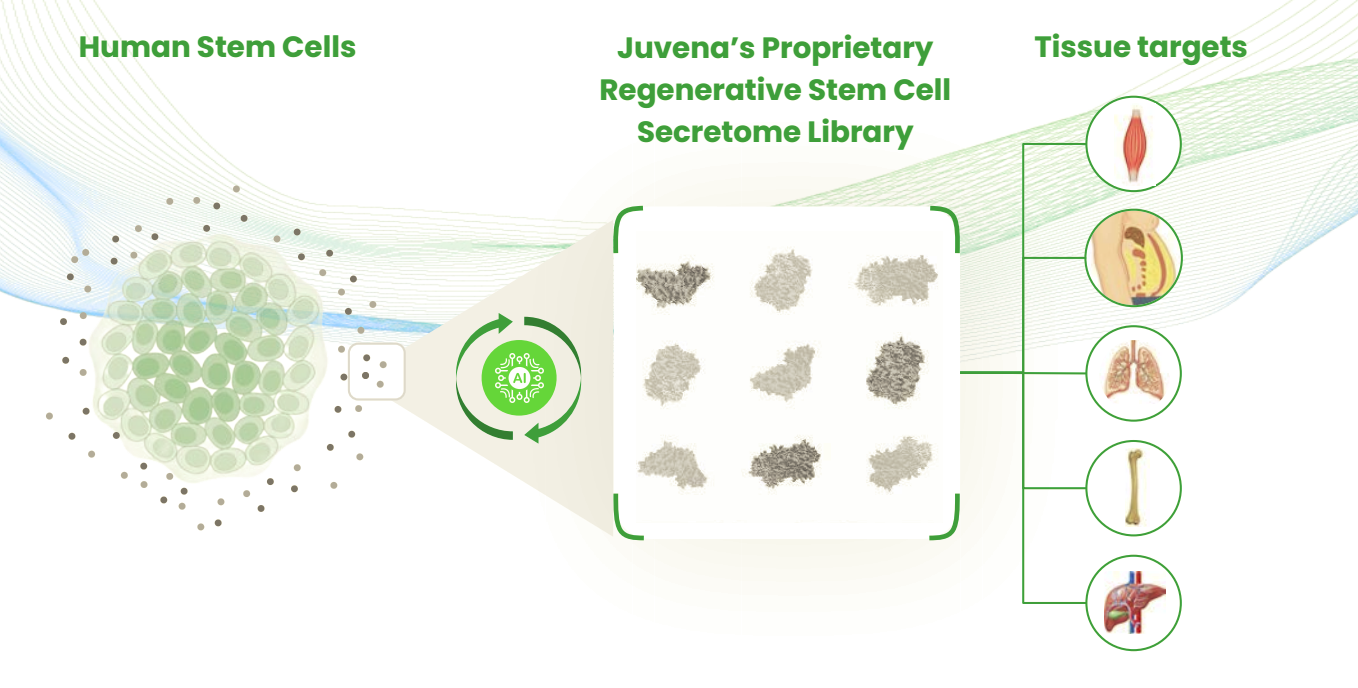
Secreted proteins act as the body’s natural signaling molecules, released by cells to communicate with their surrounding tissues and coordinate critical biological functions. They regulate immune responses, initiate tissue repair, and activate regenerative pathways across multiple organ systems. Once secreted into the extracellular space, they typically bind to receptors on neighboring cells, triggering specific downstream effects.
Among the most potent and tissue-specific signaling molecules are secreted proteins derived from human stem cells, a promising yet underexplored therapeutic class with the potential to restore cellular balance and function in diseased tissues. Juvena Therapeutics is at the forefront of this frontier. Co-founder and CEO Dr. Hanadie Yousef was among the first scientists to demonstrate that stem-cell-derived secreted proteins can rejuvenate human cells impaired by aging or disease, with published evidence across models of Alzheimer’s, osteoarthritis, and muscular dystrophy.
Building on these insights, Juvena Therapeutics developed the world’s first proprietary library of human stem cell derived secreted proteins. This library now profiles more than 2,000 unique proteins, many of which remain uncharacterized in the scientific literature. 40% of these proteins have fewer than 50 citations, and 30% have no known binding partners, creating substantial “white space” for innovation. Constructed in just 18 months, the library captures structural, receptor-binding, and tissue-expression data for each protein, forming the foundation for Juvena’s predictive drug discovery and therapeutic development strategy.
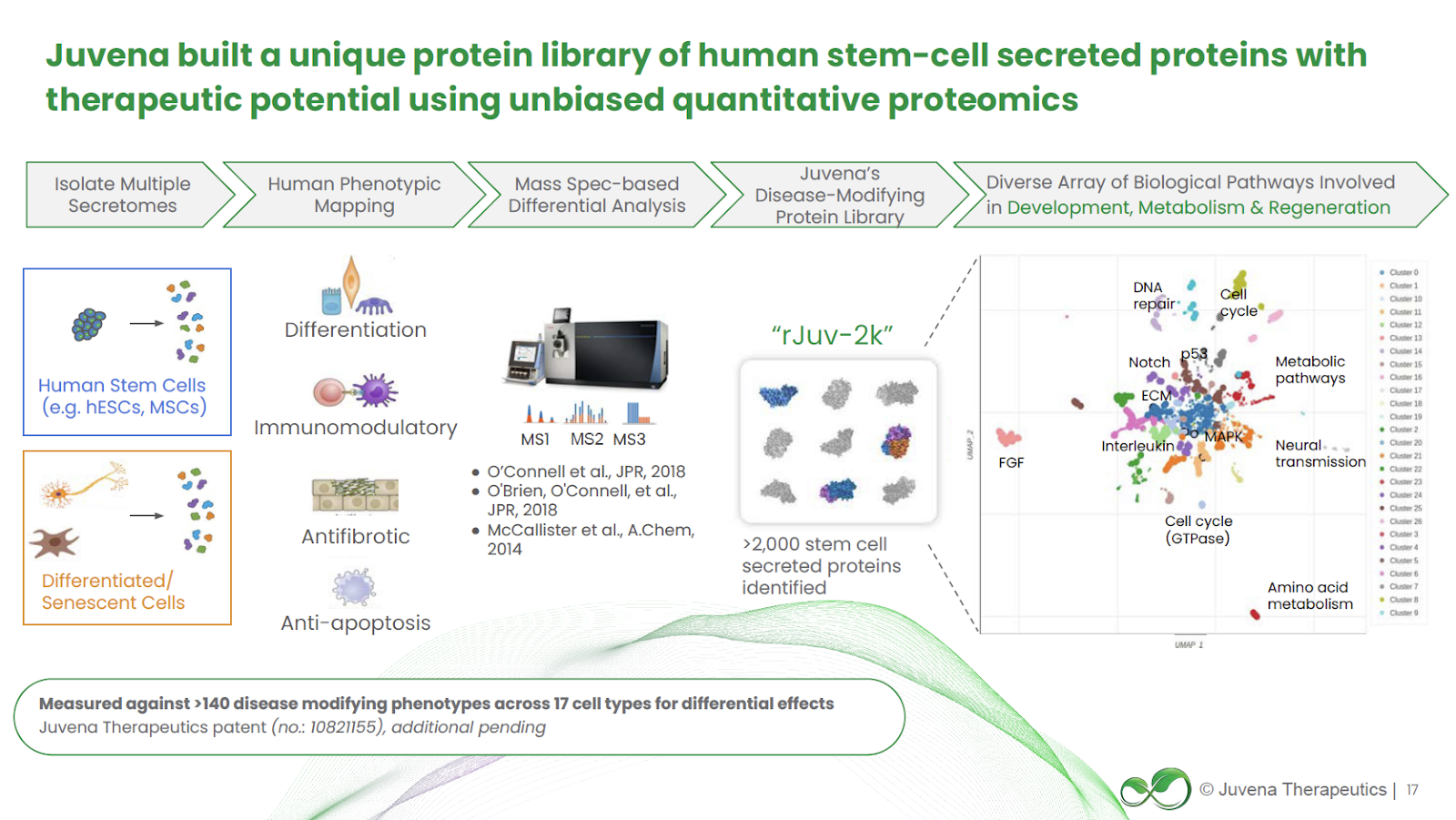
JuvNET integrates this dataset with high-throughput phenotypic screening, bioinformatics, and deep learning models to rank and predict which secreted proteins have the highest disease-modifying potential. Through iterative rounds of in vitro and in vivo validation, the platform retrains itself, improving accuracy and generating a compounding advantage with each discovery cycle. This closed-loop system allows Juvena to rapidly identify and develop first-in-class therapeutics across a broad range of rare and chronic diseases, offering a fundamentally new way to restore regenerative signaling that deteriorates with aging and disease.
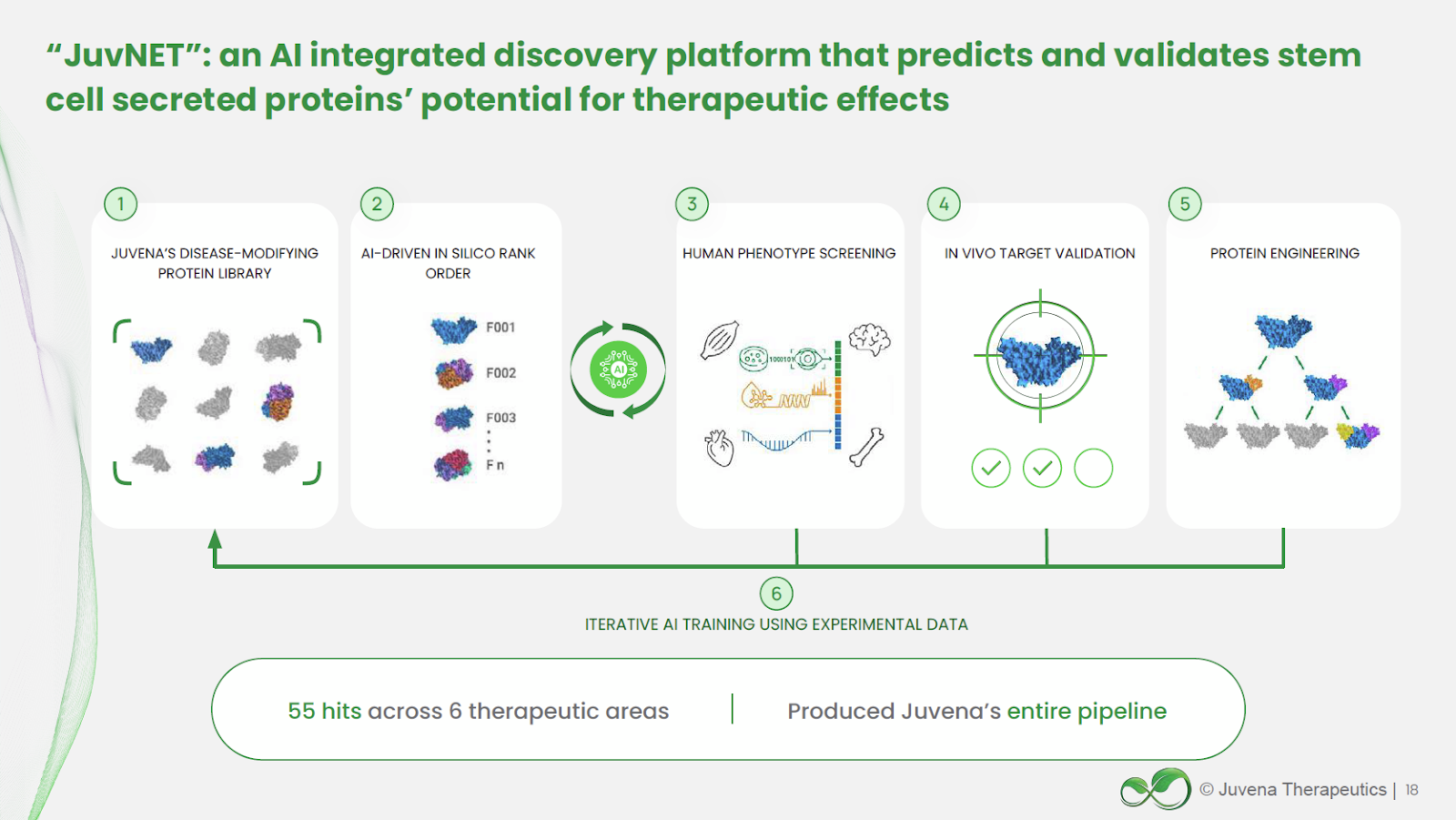
Juvena Therapeutics’s first-of-its-kind AI-powered drug discovery platform systematically identifies and develops secreted protein-based therapeutics, a largely untapped modality with powerful regenerative potential.
At the heart of this platform is a proprietary library of over 2,000 proteins secreted by human stem cells, many of which are novel or previously uncharacterized. These proteins are selected for their naturally potent, tissue-specific, and restorative properties, making them ideal candidates for safer and more effective biologics. The discovery process begins with unbiased in vitro screening in organ-specific human cell models to identify key drivers of rejuvenation for targeted tissues (e.g., muscle, liver, cartilage). Candidates showing strong efficacy progress to rigorous in vivo validation. Validated proteins are assessed for safety, manufacturability, pharmacokinetics, and mechanism of action before advancing toward clinical development.
Because these proteins are naturally produced by the body, they often require fewer chemical modifications, exhibit low toxicity, and bypass many limitations of small molecules or gene therapies. Juvena’s AI-enabled JuvNET engine has already prioritized over 300 proteins in silico, screened 22 in human cell assays, and validated 8 in animal models. Advanced protein engineering optimizes pharmacologic properties, with expansion into antibody and bispecific formats to complement core capabilities. The platform is further protected by a robust and expanding IP portfolio, including one granted patent and 14 patents pending.
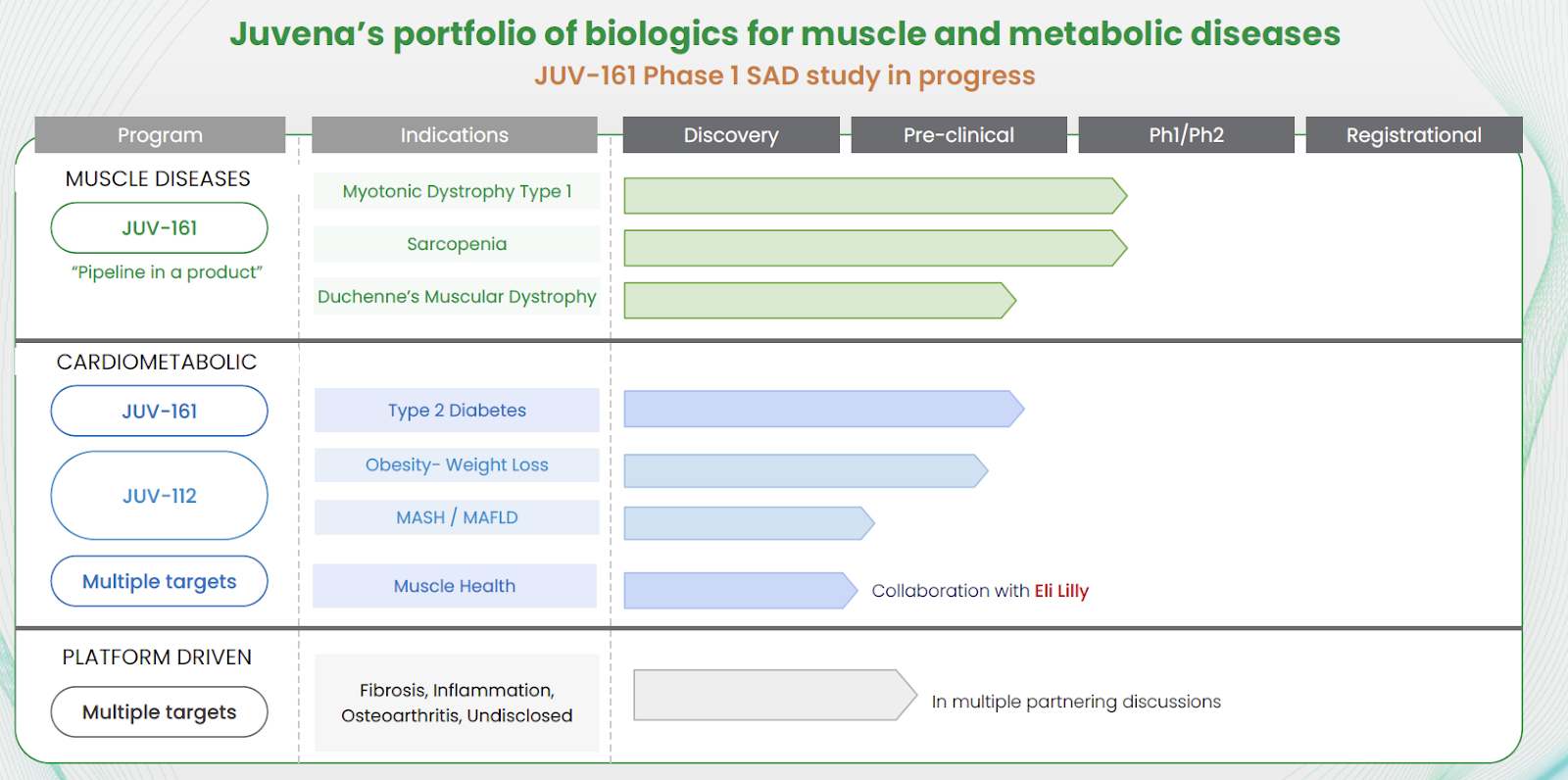
The platform is designed for continuous improvement: data from every experiment feeds back into JuvNET, increasing predictive accuracy and speed. Initial programs target neuromuscular diseases (Myotonic Dystrophy Type 1, Duchenne Muscular Dystrophy) with expansion into obesity, osteoarthritis, metabolic liver disease (MASH/MAFLD), fibrosis, and systemic inflammation.
This has allowed Juvena to already develop innovative therapeutics that target two major unmet needs, including:
(1) regenerative biologic that enhances muscle repair and metabolism to restore strength and function, with broad potential across myopathies and muscular dystrophies; and
(2) differentiated obesity therapeutic that preserves lean mass and appetite while inducing weight loss via increased energy expenditure, lipolysis, and potent anti-steatotic effects.
Preclinical data from the respective lead programs JUV-161 and JUV-112 demonstrate the platform’s translational potential, reversing muscle atrophy in neuromuscular models and promoting significant fat loss through a novel, non–appetite-suppressing mechanism.
Juvena operates a dual strategy of in-house therapeutic development and external partnerships. The company advances its own pipeline in rare and high-value chronic diseases while also licensing its discovery platform and proprietary protein library to strategic partners.
Key achievements include:
Near-term milestones (18–24 months):
Market Dynamics
Disease Burden Across Pipeline
Strategic Positioning
Juvena’s ML-powered, secreted-protein platform is distinct from traditional proteomic or gene-based approaches, offering rapid candidate discovery and validation. Competitors in proteomics have not systematically advanced secreted protein therapeutics, leaving Juvena positioned to lead.
Three Major Inflection Points
Juvena Therapeutics successfully raised a $41M Series A in November 2022, led by Mubadala Capital (sovereign wealth fund of Abu Dhabi) and Horizons Ventures, with participation from Bison Ventures, Alumni Ventures, and Jeff Dean, Head of Google AI. Plum Alley Ventures Fund I and Plum Alley Member Syndicates both also participated in this round, laying the foundation for Juvena’s emergence as the category leader in secreted protein therapeutics. Proceeds enabled the company to validate its AI-powered JuvNET discovery platform, expand its proprietary library of over 2,000 secreted proteins, and advance its two lead programs: JUV-161 (for neuromuscular regeneration) and JUV-112 (for obesity/metabolic disease) through preclinical and early clinical development.
In July 2025, Juvena closed $30M as part of an upsized $40M Series B at a pre-money valuation of $71M, once again led by Mubadala. The round included a $6M strategic investment from Eli Lilly, following its $650M multi-target discovery collaboration with Juvena focused on muscle and metabolic indications. New capital from Jefferson Life Sciences (JLS) [part of $2.5B AUM Jefferson River Capital, the family office of former President & COO of Blackstone] also joined the round with Dr. Laura Lande-Diner taking a board seat with the indication to lead a future Series C raise. This financing reflects growing institutional confidence in Juvena’s platform, pipeline, and commercial trajectory.
With over $85M in total funding to date, including $17M in non-dilutive capital from grants and partnerships, Juvena is entering a critical phase of clinical and commercial execution.
Proceeds will:
The Eli Lilly partnership provides upfront funding and potential for >$650M in milestones, further de-risking the business model and supporting future growth.

Pictured from left: Co-Founders Jeremy O'Connell, PhD and Hanadie Yousef, PhD
Hanadie Yousef, PhD, Co-Founder & CEO: An experienced scientist with a PhD from UC Berkeley, Hanadie spent 4 years at Stanford Medical School completing a postdoctoral fellowship in the Wyss-Coray lab. During this time, she published in multiple prestigious scientific journals including Nature Medicine and Cell Reports. Of particular note, Plum Alley portfolio company founder (Mammoth Biosciences) and Nobel Laureate Dr. Jennifer Doudna served on Hanadie’s PhD Committee at UC Berkeley. Hanadie has interned at Genentech and worked at Regeneron for over 5 years. In addition, she was a SPARK scholar at Stanford and earned awards at the Bay Area Aging Meeting, the Alzheimer’s Researchers’ Symposium, and the International Society for Stem Cell Research Conference.
Jeremy O’Connell, PhD, Co-Founder & CSO: Jeremy completed his PhD in Biochemistry, Cellular, and Systems Biology at UT-Austin followed by two postdoctoral fellowships at Harvard University and Medical School. During his postdoctoral fellowships, Jeremy focused on computational mapping of protein-protein interactions and mass spectrometry characterization of proteins. Jeremy has published 13 papers in journals such as PNAS.
Edward Moler, PhD, Head of Data Science: Eddie earned his PhD in Chemistry from UC Berkeley and has spent a combined 12 years at biotech companies including Novartis and Tethys Biosciences. Eddie has also managed analytics/bioinformatics departments at Phoenix Energy Technologies, Quest Diagnostics, GE Healthcare, and Spatial Genomics, Inc. In addition to his ample experience directing bioinformatics initiatives, Eddie also spent over 3 years at GE Healthcare beginning as an Analytics team leader and escalating to R&D Manager.
Jim Maguire, PhD, Head of Discovery: Jim obtained his PhD in Chemistry from University of Illinois Urbana-Champaign. After completing his postdoctoral fellowship, Jim honed R&D skills during 6 years as a manager at Ciphergen Biosciences and over 12 years at pharmaceutical company Novo Nordisk. In his spare time, Jim’s expertise was also utilized by Catalyst Biosciences as a consultant.
Juvena Therapeutics offers an opportunity to invest in a first-mover biotechnology platform targeting a large, underserved market in degenerative diseases and metabolic disorders through a novel class of secreted protein-based biologics. This modality, derived from human stem cell secretomes, offers the potential for safer, more effective, and more tissue-specific therapies than conventional small molecules, gene therapies, or antibody-based drugs. And while the platform’s scalability is validated with Eli Lilly’s commercial partnership over three years for $650M, there are considerations with this opportunity that include both market and business execution risk:
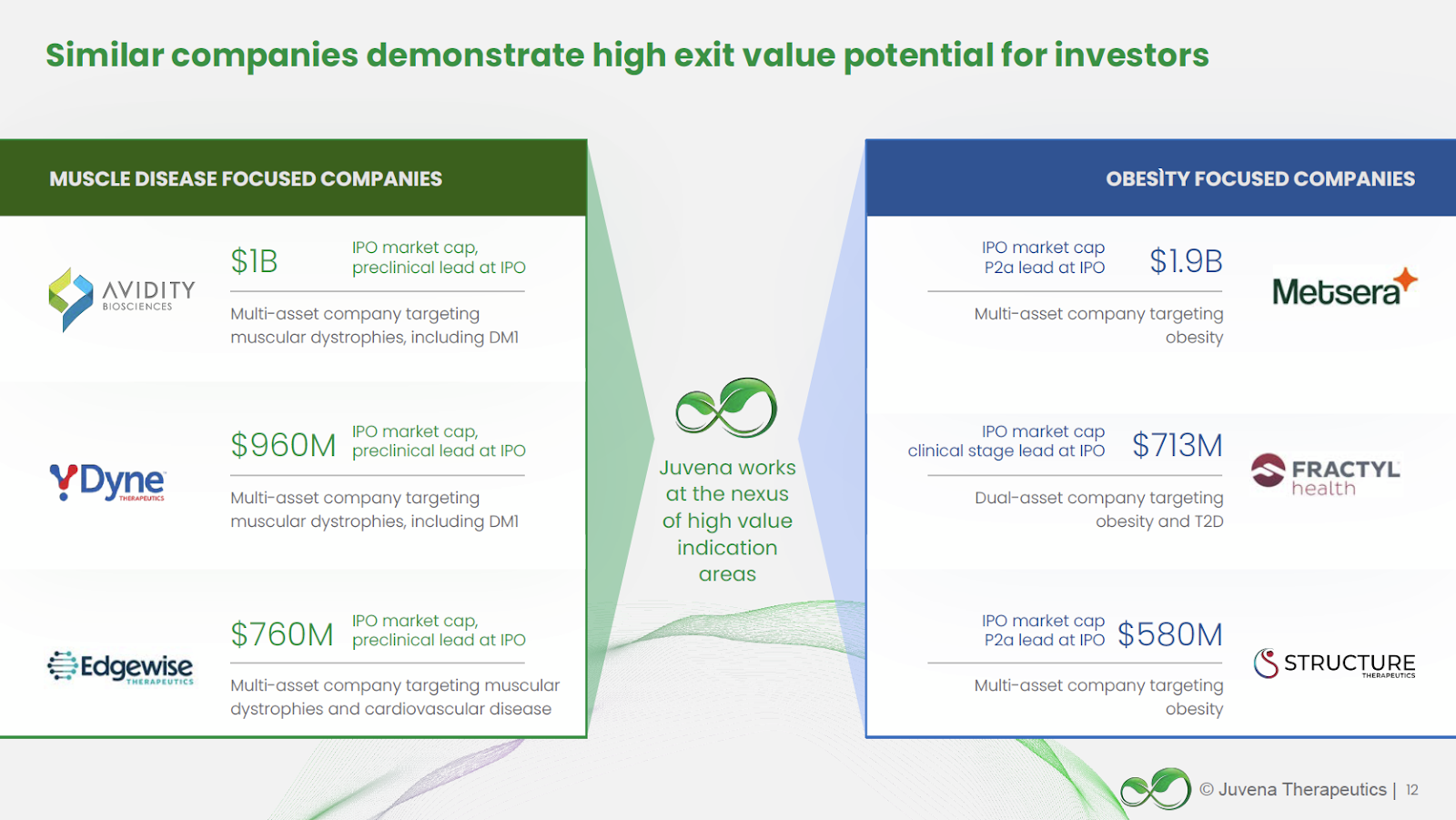
Upon discussion with the founders, Juvena put together their view of how and where the company could exit. Please see the image below for a breakdown of similar companies that have shown high exit potential for investors. The following information is presented for illustrative purposes only and contains numerous assumptions made by Juvena which may or may not occur. Investors should perform their own assessment, and are cautioned that milestones mentioned below may or may not be achieved.
Juvena compiled examples of recent market precedents and examples of IPO exit outcomes across the two markets of muscle disease and obesity focused companies. These examples with a range of preclinical and clinical assets at IPO include an exit valuation range of ~$580M at the low end to ~$1.9B+ on the high end.